This journal entry is dedicated to planetary atmospheres, since this is probably the most difficult part of a worldbuilder’s journey. Of course, you can have things easier way, say, if you have earthlike conditions or you have additional data laid out for you in case of a standard star. But what if things were different?
I’m going to explain each step, adding some theoretical info as well. The Four Elements series will cover topics such as the atmosphere, the ocean, geological activity, insolation and planetary climate. And maybe something else I’ll find necessary to add.
The quest doesn’t start where one might think it does – not on a planet, but on a star. So, long before we can model our planetary atmosphere or anything else related to it, first we should consider stellar spectrum. There are several ways of getting it: taking a real detailed spectrum for existing star or computing a synthetic stellar spectrum. It all depends on your star of choice and how deep you are willing to go into details.
Why is this important? To design your own exclusive and unique planetary atmosphere model: maybe the air is so thin at altitude of 5 km, so your local people will never go to the mountains, for example. Whatever it is, you’ll get the full set of necessary parameters, some of which you might have had imagined, and some of which you probably didn’t expect at all. Anyway, you will get to know your planet better.
Don’t be afraid of experimenting with things. They are not as hard or incomprehensible as might appear to be.
Planet atmosphere
The atmosphere is an envelope of gas mixture around the planet. It is held down by gravity, and the weight of that gas is pressure (as in mass times g). The total pressure is the sum of partial pressures of gasses in the mixture. The partial pressure is the contribution of a particular gas constituent to the total pressure, and is found as the total pressure times the volume fraction of gas component.
The proportion of gases found in the atmosphere changes with altitude. Distinct layers (such as troposphere, stratosphere, etc.) are identified using thermal characteristics, chemical composition, molecule movement, and density.
Individual molecules are moving freely in gas and if their motion velocity exceeds the planet’s escape velocity, the molecules will escape into space from the outer edge of the atmosphere. A certain amount will always exceed escape velocity, and if that percentage is too high, the atmosphere will leak away in a geologically short term. Thus, enough gravity is necessary to hold the atmosphere. The outer atmosphere temperature plays a vital role in this process as well, since gas molecules travel faster with increasing temperature. The hotter the exosphere is, the greater gravity must be. To keep things in balance, worlds closer to their stars must be larger to hold atmospheres equivalent to those around cooler worlds. Thus, atmospheric composition is also important, because lighter molecules move faster at the same temperature. Same surface gravity can keep one molecules, but can’t hold others; in case of Earth hydrogen and helium are too light for our gravity.
Composition and pressure are not completely free parameters, though. They are influenced and modified by chemical reactions with the surface of the planet (e.g. atmosphere interaction with crustal rocks over time in the carbonate-silicate cycle), living things and photodissociation (stellar UV light breaks up the hydrogen-bearing compounds like water, ammonia and methane) at the outer edge of the atmosphere. The atmosphere changes over geological time along with the evolution of the star, life and loss of lighter gasses.
Terrestrial-like planets may obtain atmospheres from three primary sources: capture of nebular gases, degassing during accretion, and degassing from subsequent tectonic activity. While capture of gases is vital for gas giants, low-mass terrestrial planets are unable to capture and retain nebula gases, which also may have largely dissipated from the inner solar system by the time of final planetary accretion. Atmospheric mass and composition for terrestrial planets is therefore closely related to the composition of the solid planet (Elkins-Tanton & Seager, 2008a).
In case of humans and animals the atmosphere has limits on its composition. To be breathable, it must have levels of molecular oxygen (O2) between 0.16 and 0.5 atm; higher concentrations of oxygen are toxic (severe cases can result in cell damage and death), lower than minimum are not enough to support human life. Hypoxia (oxygen deprivation) and sudden unconsciousness becomes a problem with an oxygen partial pressure of less than 0.16 atm. Hyperoxia (excess oxygen in body tissues), involving convulsions, becomes a problem when oxygen partial pressure is too high. Our present atmosphere contains 21% molecular oxygen (partial pressure of 0.21 atm).
Also, to prevent nitrogen narcosis under high pressures (the diver’s “rapture of the deep”) the partial pressure of nitrogen (N2) must be less than 3 atm.
As for other toxic stuff, the level of carbon dioxide must be less than 0.02 atm to breathe indefinitely, and less than 0.005 atm to avoid physiological stresses. In case of CO2 concentration above normal levels the only habitable places for humans might be high regions, like mountains. But then again, too little oxygen higher up can be troublesome.
Many plants, however, can survive and thrive in low oxygen-high CO2 environment. Earth’s plants will grow in many atmospheres that are unbreathable to humans and animals, unless the runaway greenhouse ruins the place completely (like Venus).
By building planet atmosphere and climate models you can see what are the boundaries for life under different stars and atmospheres. How fast the atmosphere thins upward? What are the properties of layers (altitudes, temperatures, pressures, composition, ozone layer (ozone is also toxic), gravitational pull, etc.)? What is the climate and weather pattern? The broader applications for the model include your planet aerospace or colonization/terraforming history, if applicable. The thickness of the atmosphere has some consequences. The thicker it is (and/or the lower the gravity), the easier flight is. Sound also travels better in a denser medium. Storms can be more intense if mass of moving air is greater.
More detailed description of atmospheres is beyond the scope of this article, but can be found on the Internet or in textbooks. In fact, if you know little about how atmospheres work, further reading into subject is required before building anything. Some useful book titles are listed in my LibraryThing catalogue, which is constantly growing. My goal here is to describe the tools: what data for models is required and how those models can be used to produce desirable results.
Climate dynamics model
The MITgcm (MIT General Circulation Model) is a numerical model designed for study of the atmosphere, ocean, and climate dynamics. MITgcm is freely available to all and can be run on a home pc or laptop, and is enough to play with your planet in detail.
There are some other models, such as NASA/GISS 4×3 Atmosphere-Ocean Model or the Community Earth System Model (CESM).
Running the NASA/GISS model requires a significant investment in time and money, and it is designed to run on multiprocessor machines. It can reproduce the seasonal and regional mean values and variations of climate quantities such as temperature, pressure, precipitation, cloud cover, and radiation with reasonable degrees of precision, and many other things. I do not recommend this one for our purpose (though, if you own a multiprocessor workstation, you can try).
The CESM is also designed for simulating Earth’s climate system, but, as with the NASA/GISS, it is not simple at all. It is a coupled climate model composed of five separate models simultaneously simulating atmosphere, ocean, land, land-ice, and sea-ice.
However, before you can model weather and climate, an atmospheric layered model is required. You can take earthlike model (e.g. Standard Atmosphere) or you can make your own. For the latter purpose you’ll need another piece of code, described in the section below.
Photochemical and radiative/convective atmosphere models
The coupled photochemical and radiative/convective atmosphere model was used to study earthlike planets around different types of stars: F2V, G2V (Sun), K2V (Segura et al., Astrobiology, 2003) and M stars (Segura et al., Astrobiology, 2005); Grenfell et al. 2006, 2011; Kasting et al. 1996.
This model requires stellar spectrum, which can be taken from the database or synthesized. Some spectra are hard to find. The ones used by Segura et al. can be taken from the VPL site.
Stellar flux greatly influences chemical processes in the atmosphere and biological processes on the planet. Each star has its individual flux “signature”.
In Segura’s model the “Earth” is assumed to be at a distance equivalent to 1 AU in the extrasolar planet system. The orbital radius is scaled according to stellar luminosity, and the planet is then moved inward or outward until its calculated surface temperature is 288 K. Also, the term “mixing ratio” has the same meaning as “mole fraction”.
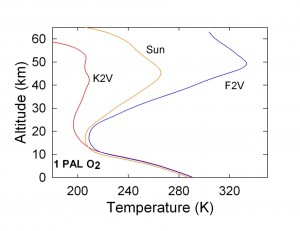
The planet around the F star develops a thicker ozone layer because of the abundance of short-wavelength UV radiation (lambda < 200 nm) that can dissociate molecular oxygen.
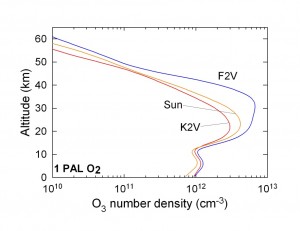
The surface UV flux increases with decreasing partial pressure of O2, but the behavior is very nonlinear. Good UV shield develops above 10^-2 of present atmospheric level of O2.
M stars emit very little near-UV radiation (200-300 nm), but active M (and, in fact, early K) stars emit lots of UV radiation shortward of 200 nm (chromospheric emission). One can therefore split molecular oxygen (and, hence, make ozone), but the ozone photochemistry is very different. Methane in Earth’s atmosphere is mostly destroyed in chemical reactions triggered by UV-flux at 310 nm. In atmospheres near M stars the lifetime for methane is long.
Synthetic stellar spectrum
If you have a star type that is not on the VPL list of spectra, acquiring a synthetic spectrum is where you’ll have to start building your model. There are numerous ways and software packages to compute a synthetic spectrum, but the easiest one is to use the BaSeL interactive server: it saves time and sanity. This tool presents a user-friendly interface of an interpolation engine, that allows on-line computations of synthetic stellar spectra for any given set of fundamental parameters Teff, log g and [Fe/H]. More info about BaSeL is found in “The BaSeL interactive web server: a tool for stellar physics”. Please note that fundamental parameters are taken from the real star’s data or computed stellar evolution model.
# Have fun and more modeling to follow.

I’m really glad to have found your blog. These worldbuilding posts have been really useful for me.
In the past I have tried and given up on the NASA/GISS and CESM models and I have played with simpler educational GCMs. NASA/GISS and CESM were just well beyond my capabilities. Both computer assets and apparently intellectual capacity.
From what you’re saying, the MITGCM is reasonably easy to deal with(as such things go), so I’m feeling a bit mentally underendowed that I can’t get the thing running. Perhaps at some point you could post a run-through of setting up and running the gcm. “MITGCM for Dummies” would be even better, but at least I, personally, would really appreciate a basic run-down. I know tutorials and docs exist for the program, and maybe once I can get a mental foothold on running the thing they’ll start to make more sense, but it’s difficult to find usage instructions hidden in all the physics textbook elements.
Hi Astrographer, thanks for dropping by.
I haven’t been doing any modeling lately because I’m working on my trilogy of novellas now. There’s a lot of writing going on. I will have a pause before I will dive into writing novels to get to it.
I plan to run a planetary atmosphere/ocean MITgcm model some time (hopefully) next year, since I need the output for the novels. I have to decide on (read: calculate) quite few things for my fictional planet before putting them into my model. When I’m done, I will post my detailed steps and results here on my blog.
MITgcm only seems simple, but in reality it is rather complicated if you are not dealing with ocean/atmosphere science professionally, since there is a lot of “tuning” to do.
I suggest using Ubuntu 10.10 or another version of Linux for the run. If you are good with Linux, no big deal here. If not, you will need an dual boot/clean installation or a virtual machine in any case.
Cheers, J